FDA Approves First Alzheimer's Blood Test Under Biden Administration
Regulatory decision highlights ongoing debate over laboratory test oversight
The Food and Drug Administration has authorized Japan-based Fujirebio to market the first blood test for diagnosing Alzheimer's disease in certain patients. The test, which measures proteins associated with amyloid plaques in the brain, is intended for adults 55 and older who show signs of cognitive decline. FDA official Dr. Michelle Tarver called the clearance "an important step for Alzheimer's disease diagnosis," potentially making testing more accessible for U.S. patients earlier in the disease progression.
The FDA's authorization comes amid a broader regulatory debate that developed during the Biden administration. In 2023, the FDA had attempted to implement stricter oversight of laboratory-developed tests, including those for Alzheimer's diagnosis, arguing that insufficient validation could put patients at risk. The agency cited concerns about tests being marketed without proper authorization, particularly when treatable conditions may have symptoms similar to Alzheimer's or other forms of dementia.
KEY POINTS
- •FDA approves Alzheimer's blood test
- •Part of Biden-era regulatory efforts
- •Court struck down broader FDA rules
However, the FDA's regulatory efforts faced significant pushback. A lawsuit supported by the American Clinical Laboratory Association successfully challenged the new rules, with a federal judge in Texas overturning the regulation in March 2025. The association celebrated the ruling as a victory that "protects patient access to critically needed testing services" and removes what they described as burdensome regulations that would have undermined the clinical laboratory system.
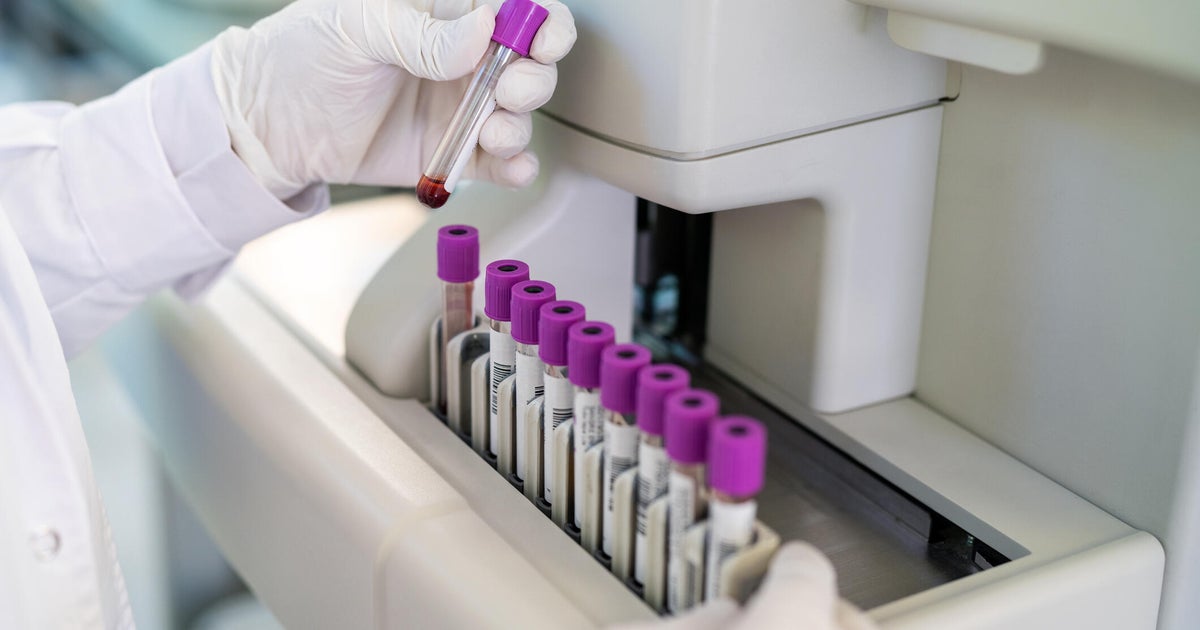
Fujirebio's newly approved test demonstrated high accuracy in clinical studies, with over 90% of positive results matching confirmatory tests like brain scans or spinal fluid analysis. The test works with the company's Lumipulse equipment, which is described as already widely available in U.S. clinical laboratories. The automated system can process up to 120 tests per hour on blood and other samples, potentially expanding access to earlier diagnosis when interventions may be more effective.
The approval represents a significant development in Alzheimer's diagnostics, occurring during the transition between the Biden and Trump administrations. While other blood tests for Alzheimer's have been available for years without this type of FDA approval, Fujirebio's test has now received official marketing authorization through the standard regulatory pathway. This development comes as the healthcare system continues to seek improved methods for early detection and treatment of neurodegenerative conditions affecting millions of Americans.